Ethylene glycol
Ethylene glycol
CAS: 107-21-1
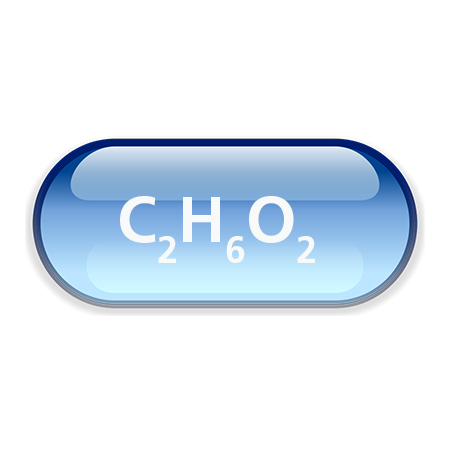
Chemical formula: C2H6O2 or C2H4(OH)2
Synonyms: Glycol, ethanediol, monoethylene glycol
Resistance: +20°C= suitable, +60°C= suitable
Density: 1.1132 g/cm³ @ Temp: 20 °C
Usage
Ethylene glycol is mainly used in antifreeze for cars, for example, but also in solar thermal collectors. With the low freezing point, it can also be used for de-icing aircraft or windscreens.
In radiators on cars, glycol has two functions, partly as antifreeze for the cooling system, and partly as rust protection in the cooling system. A mixture of 50 percent water and 50 percent glycol gives a freezing point of about -40 °C while raising the boiling point to about 108 °C. The excess pressure in a car's cooling system also means that the liquid boils at approximately 120 °C. The elevated boiling point allows a car to be driven at higher temperatures and therefore have more efficient combustion.
Glycol freezes at -13 °C[3] while water freezes at 0 °C. It is thus the water-glycol mixture that gives the desired low freezing point.
Ethylene glycol is also used in indirect cooling and heat pump systems.
Storage - Ethylene glycol
Store cool and dry, in well-ventilated areas. Do not store in direct sunlight.
Dosage/Mixture - Ethylene glycol
The vapours are heavier than air and can spread along floors over a large distance. Avoid inhalation of vapour/mist.
Trademarks - Ethylene glycol
....
Pipes
PE, PP
Gaskets
EPDM
NB
Harmful if swallowed. May cause organ damage through prolonged or repeated exposure
